Research
Phase transitions, instabilities and patterns in biological systems
As theoretical physicists we are interested in the basic principles which underly the patterns and the morphology in various kinds of biological systems with a strong focus on the organization of the cellular cytoplasm and the evolution of cells at the origin of life. By applying concepts from non-linear dynamics, non-equilibrium thermodynamics, kinetic theory and elasticity theory we look for the minimal ingredients necessary to describe the dynamics of these systems.
Our fascination for biological systems is based on their inherent local consumption and dissipation of energy, which can power chemical reactions or give rise to active force generation on the scale of the constituent particle. These active processes can drive changes of the state of biological matter or affect its shape and even its mechanical integrity. In particular, we are intrigued by biological systems, which can abruptly change their degree of order: Examples range from the spontaneous emergence of collective motion (swarming), over the controlled positioning of intra-cellular droplets in maintained concentration gradients, to the aggregation kinetics of proteins inside cells. The change in degree of order can often be understood as phase transitions. Phase transitions can cause dramatic changes in chemical and physical properties of biological matter. These changes often require little energy input to perturb or quench the system and in general there is no need to supply the energy costs to rearrange each molecule. We are fascinated by the possibility that living systems may control the occurrence of phase transitions in order to maintain or switch biological functions.
Phase transitions in cells
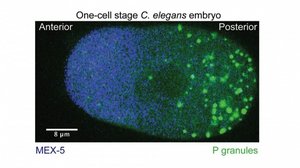
Cells have to control and switch biological function. In some cases this is achieved by the formation of chemically distinct compartments. Recent evidence suggests that there is a class of compartments, which can be regarded as liquid-like droplets formed by phase separation from the cellular cytoplasm. Together with Prof. Frank Jülicher (Max-Planck Institute for the Physics of Complex Systems) and Prof. Anthony Hyman (Max Planck Institute of Molecular Cell Biology & Genetics) we investigate the question of how cells use liquid phase separation to form chemically distinct non-membrane bound compartments inside cells and how cells control the position of these compartments in space using concentration gradients of regulating proteins [Annual Reviews, 30: 39–58]. In the specific case of the C. elegans embryo we could understand the mechanistic principles of how the MEX-5 protein concentration gradient spatially controls phase separation of P granule compartments [Cell, 6, 166: 1572–1584]. From a theoretical perspective, the concentration gradient leads to a spatially inhomogeneous scenario of droplet ripening, which can be described by an extension of the classical ripening theory of Lifschitz & Slyozov [Read More]. In addition, we found that the switch of position can be understood as a first order phase transition [Read More]. Further interests are devoted to the influence of chemical reactions and external control parameters such as temperature and pH on the stability and dynamics of these liquid-like compartments. The presence of chemical reactions can affect the stability of liquid-like drops and even drive the division of droplets [Read More]. This finding indicates that liquid droplets combined with chemical reactions might have played a fundamental role in the evolution of protocells. The stability of liquid-like compartments is also influenced by changes of pH, which affects the interactions between components and can thereby drive phase separation in region where the pH is close to the isoelectric point (manuscript in preparation).
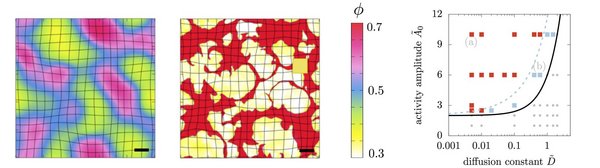
Differential-activity driven instabilities in biphasic active matter
Biological systems are distinguished by the presence of active stresses which can affect their physical properties and alter their stability. For example, active stresses give rise to collectively moving streaks and clusters, rotating ring, swirl or aster-like patterns, or the remodelling of cell-to-cell junctions in living tissues. These systems are typically described as a single phase with active stresses that drive the assembly of the constituents and the properties of the phases are typically assumed as liquid-like or even gases.
However many active material cannot be treated as fluids. Examples include cartilage, bone, tissues in early development, and superprecipitated systems such as networks of filaments connected by crosslinks and molecular motors. The presence of activity in these systems can drive the macroscopic contraction of gels, the compaction of cells during the condensation of cartilage cells, the network formation of osteoblasts during skull closure in embryos, and the formation of furrows in tissues. This requires the augmentation of previous passive biphasic descriptions, such as associated with poroelasticity to account for active stress regulation and diffusion in cells and tissues. While recent work has included activity in a poroelastic description, the material was assumed to be homogeneous and stable despite active stress generation in one of the phases. Here we question this assumption of stability of an active mixture composed of two phases with different mechanical properties, and ask under what physical conditions an active poroelastic material might contract/condense or disintegrate/fragment, a phenomenon seen in a variety of experimental systems.
In a collboration with Chris H. Rycroft we found that differential activity between the solid and fluid phases that constitute an initially homogeneneous poroelastic medium can drive a mechanical instability leading to the demixing of a homogeneous medium into condensed solid-like patches that arise due to macroscopic contraction, reminiscent of observations in superprecipitated gels and compacted cells in tissues. Depending on the rate and ability of transport of material and stress in the biphasic material, we find both uniformly growing and pulsatile instabilities leading to assembling, disassembling and drifting solid-like clusters that undergo fusion and fission (see movies below).